About Fuel Cells:
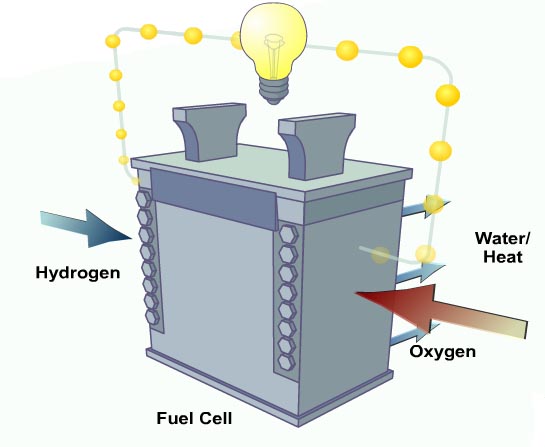
A fuel cell is an electrochemical conversion device. It produces electricity from fuel (on the anode side) and an oxidant (on the cathode side), which react in the presence of an electrolyte. The reactants flow into the cell, and the reaction products flow out of it, while the electrolyte remains within it. Fuel cells can operate virtually continuously as long as the necessary flows are maintained.
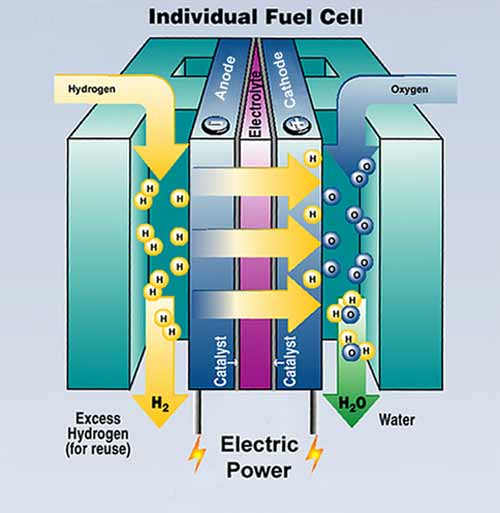
Display of components of an individual fuel cell.
Fuel cells are different from electrochemical cell batteries in that they consume reactant from an external source, which must be replenished -- a thermodynamically open system. By contrast batteries store electrical energy chemically and hence represent a thermodynamically closed system.
Many combinations of fuel and oxidant are possible. A hydrogen cell uses hydrogen as fuel and oxygen (usually from air) as oxidant. Other fuels include hydrocarbons and alcohols. Other oxidants include chlorine and chlorine dioxide.
History:
The principle of the fuel cell was discovered by German scientist Christian Friedrich Schönbein in 1838 and published in one of the scientific magazines of the time.[11] Based on this work, the first fuel cell was demonstrated by Welsh scientist Sir William Robert Grove in the February 1839 edition of the Philosophical Magazine and Journal of Science and later sketched, in 1842, in the same journal. The fuel cell he made used similar materials to today's phosphoric-acid fuel cell.
In 1955, W. Thomas Grubb, a chemist working for the General Electric Company (GE), further modified the original fuel cell design by using a sulphonated polystyrene ion-exchange membrane as the electrolyte. Three years later another GE chemist, Leonard Niedrach, devised a way of depositing platinum onto the membrane, which served as catalyst for the necessary hydrogen oxidation and oxygen reduction reactions. This became known as the 'Grubb-Niedrach fuel cell'. GE went on to develop this technology with NASA and McDonnell Aircraft, leading to its use during Project Gemini. This was the first commercial use of a fuel cell. It wasn't until 1959 that British engineer Francis Thomas Bacon successfully developed a 5 kW stationary fuel cell. In 1959, a team led by Harry Ihrig built a 15 kW fuel cell tractor for Allis-Chalmers which was demonstrated across the US at state fairs. This system used potassium hydroxide as the electrolyte and compressed hydrogen and oxygen as the reactants. Later in 1959, Bacon and his colleagues demonstrated a practical five-kilowatt unit capable of powering a welding machine. In the 1960s, Pratt and Whitney licensed Bacon's U.S. patents for use in the U.S. space program to supply electricity and drinking water (hydrogen and oxygen being readily available from the spacecraft tanks).
United Technologies Corporation's UTC Power subsidiary was the first company to manufacture and commercialize a large, stationary fuel cell system for use as a co-generation power plant in hospitals, universities and large office buildings. UTC Power continues to market this fuel cell as the PureCell 200, a 200 kW system (although soon to be replaced by a 400 kW version, expected for sale in late 2009). UTC Power continues to be the sole supplier of fuel cells to NASA for use in space vehicles, having supplied the Apollo missions, and currently the Space Shuttle program, and is developing fuel cells for automobiles, buses, and cell phone towers; the company has demonstrated the first fuel cell capable of starting under freezing conditions with its proton exchange membrane automotive fuel cell.
Fuel Cell Applications:
Fuel cells are very useful as power sources in remote locations, such as spacecraft, remote weather stations, large parks, rural locations, and in certain military applications. A fuel cell system running on hydrogen can be compact and lightweight, and have no major moving parts. Because fuel cells have no moving parts and do not involve combustion, in ideal conditions they can achieve up to 99.9999% reliability. This equates to around one minute of down time in a two year period.
Micro combined heat and power systems such as home fuel cells and cogeneration for office buildings and factories are in mass production phase. The stationary fuel cell application generates constant electric power (selling excess power back to the grid when it is not consumed), and at the same time produces hot air and water from the waste heat. A lower fuel-to-electricity conversion efficiency is tolerated (typically 15-20%), because most of the energy not converted into electricity is utilized as heat. Some heat is lost with the exhaust gas just as in a normal furnace, so the combined heat and power efficiency is still lower than 100%, typically around 80%. In terms of exergy however, the process is inefficient, and one could do better by maximizing the electricity generated and then using the electricity to drive a heat pump. Phosphoric-acid fuel cells (PAFC) comprise the largest segment of existing CHP products worldwide and can provide combined efficiencies close to 90% (35-50% electric + remainder as thermal) Molten-carbonate fuel cells have also been installed in these applications, and solid-oxide fuel cell prototypes exist.
The world's first certified Fuel Cell Boat (HYDRA), in Leipzig/Germany
Since electrolyzer systems do not store fuel in themselves, but rather rely on external storage units, they can be successfully applied in large-scale energy storage, rural areas being one example. In this application, batteries would have to be largely oversized to meet the storage demand, but fuel cells only need a larger storage unit (typically cheaper than an electrochemical device).
One such pilot program is operating on Stuart Island in Washington State. There the Stuart Island Energy Initiative has built a complete, closed-loop system: Solar panels power an electrolyzer which makes hydrogen. The hydrogen is stored in a 500 gallon tank at 200 PSI, and runs a ReliOn fuel cell to provide full electric back-up to the off-the-grid residence. The SIEI website gives extensive technical details.

